eazyplex® Candida auris
Avoid ineffective therapy and hygiene management!
Direct diagnosis from swabs or culture confirmation in just 25 minutes.
“Candida auris poses a risk to patients in healthcare settings across Europe due to its propensity to cause outbreaks and its resistance to antifungal drugs. Difficulties in laboratory identification and a lack of awareness of this Candida species can delay early detection and reduce the potential increase for a horizontal transfer. C. auris was first identified in 2009 and within a few years has become a cause of healthcare-related infections. Outbreaks have been reported in countries on five continents. The number of reported C. auris cases in European countries has increased significantly since the last ECDC rapid assessment of the risk of C. auris in December 2016. There remains a need to raise awareness of C. auris in European healthcare settings so that they can adapt their laboratory testing strategies and implement improved infection prevention and control measures where appropriate.”
European Center for Disease Prevention and Control. Candida auris in healthcare settings - Europe - first update, 23 April 2018. Stockholm: ECDC; 2018
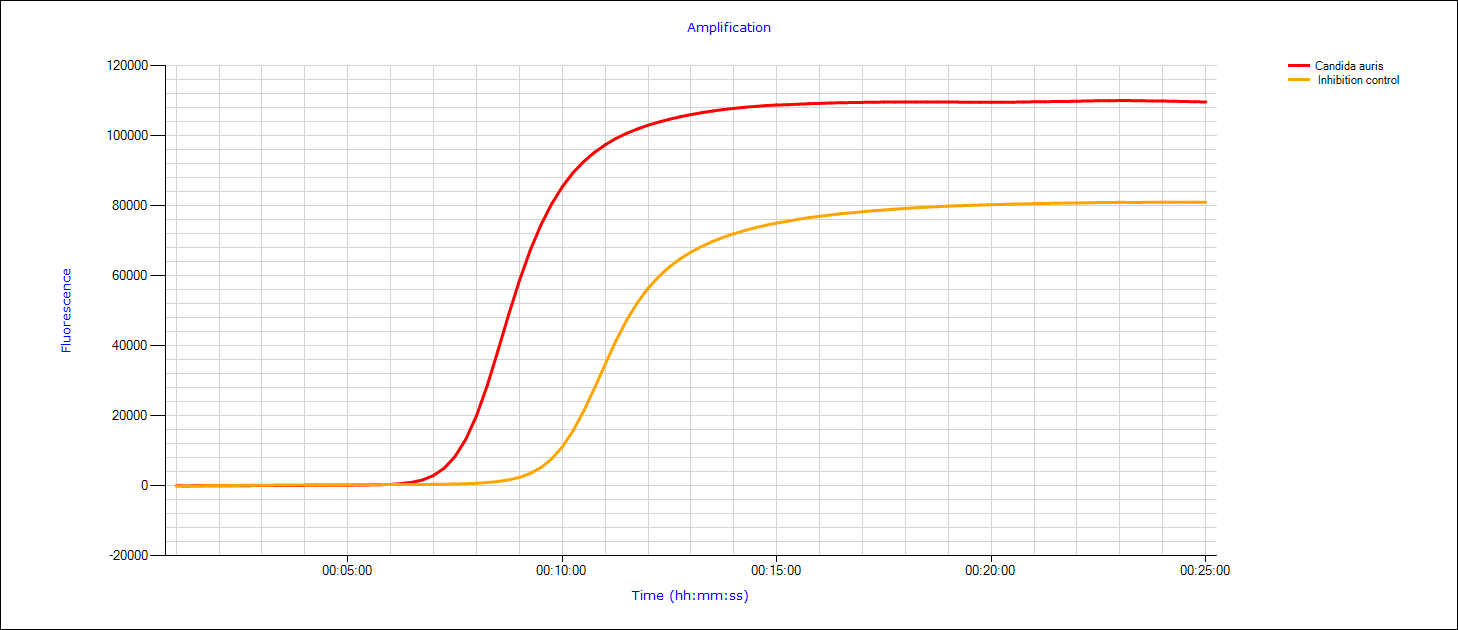
The graph of the detection of a Candida auris is shown. The detection directly from the sample (swab) is reported here as positive after 7:35 minutes.
Candida auris a worldwide risk for hospital patients
First discovered in Japan (in a patient's eponymous ear) in 2009, Candida auris was recognized by the US Centers for Disease Control and Prevention (CDC) in August 2017 and by the European Center for Disease Prevention and Control (ECDC) in April 2018 global or Europe-wide risk for hospital patients. This was justified by the property of this pathogen not only to lead to outbreaks in medical departments, but also by the multi-resistance of the fungus and the problems of correctly identifying it with classic laboratory methods. Due to this unfortunately still incomplete standard diagnostics, there is always missing or delayed identification and thus corresponding ineffective therapy decisions. This in turn leads to the acceleration of the outbreak process. What's more, unlike all other Candida species, Candida auris is regularly transmitted from patient to patient, with intermediate stations such as hands, surfaces or medical equipment often paving the way. This is very unusual for Candida species. Commonly, endogenous pathways (from the colonized intestinal tract) lead to infection in related species such as C. albicans and C. glabrata. It is assumed that smear infections cause the spread and rapid infection of other patients with Candida auris. Spread through the air we breathe, as is often the case with cold viruses, can be ruled out based on current information. Since 2013, a total of 620 cases in seven countries (Spain, United Kingdom, Germany, France, Belgium, Norway, Austria) have been sent to the ECDC. 75% of these cases remained with harmless colonization, but in 25% colonization ended with sepsis or other infection. The US health authority CDC estimates that between 30 and 60 percent of all infections in which Candida auris enters the body are fatal. A significantly increasing number of cases was determined in 2016 and 2017, probably due to the developing and improved diagnostic systems. The largest nosocomial outbreak to date occurred in 2015/2016 in a cardiac surgery department in London. All experts, from the German National Reference Center for Invasive Fungal Infections (NRZMyk), the United States Health and Medical Administration (CDC) and the European Center for Disease Prevention and Control (ECDC), agree that hospitals include Candida auris in their hygiene plans and processes for dealing with this pathogen should be established. This should result in raising awareness of C. auris in European healthcare facilities so that they can adjust their laboratory testing strategies and implement improved infection prevention and control measures where appropriate.



